Pourquoi les batteries lithium-ion dominent-elles le marché ?
Les batteries sont composées d’accumulateurs. Les batteries ont la particularité de stocker de l’énergie et de pouvoir être rechargées. Elles comportent des cellules, chacune dotée d’une électrode positive (la cathode), d’une électrode négative (l’anode), d’un séparateur et d’un électrolyte qui délivre de l’énergie électrique en changeant d’état. Selon les composants chimiques et les matières utilisées pour ces éléments, les propriétés de la batterie sont différentes et ont un impact sur la quantité d’énergie stockée et délivrée, la puissance fournie ainsi que sur le nombre de cycles de charges et de décharges possibles. Les fabricants de batteries recherchent constamment des systèmes électrochimiques plus économiques, plus denses, plus légers et plus puissants.

Un peu d’histoire
En 1786, le biologiste Luigi Galvani disséquait une grenouille. A chaque fois que le scalpel en acier de Galvani touchait un crochet en cuivre, qui tenait en place la cuisse de la grenouille, celle-ci se contractait. Galvani pensa que cette énergie provenait de l’animal et l’a donc appelée « électricité animale ». Alessandro Volta, associé de Galvani, n’était pas d’accord avec cette idée. Il était convaincu que l’électricité avait été générée par les deux différents métaux dans un milieu humide. Des expériences ont ensuite confirmé cette idée et, en 1797, Volta conçut la première batterie : la pile voltaïque. Elle était composée de 49 paires de disques de cuivre et de zinc, disposés alternativement, séparés par un tissu trempé dans de l’eau salée. Mais dans une pile voltaïque, l’électricité est générée par une réaction chimique et lorsqu’elle est épuisée, la pile ne peut pas être rechargée.
En 1899, Waldmar Jungner, un Suédois, inventa la batterie au nickel-cadmium. En 1947, Neumann réussit à étanchéifier complètement l’accumulateur. Ces progrès conduisirent à la batterie moderne étanche au nickel-cadmium.
Les premières batteries primaires au lithium, sans recharge, apparurent au début des années 1970. Des tentatives de développement de batteries rechargeables au lithium suivirent dans les années 1980 mais échouèrent à cause de problèmes de sécurité. Du fait que le lithium sous forme métallique est par nature très instable, particulièrement lors de la charge, la recherche s’est ensuite orientée vers des batteries à lithium non métallique utilisant des ions de lithium. Bien que légèrement inférieur en densité d’énergie que son homologue à lithium métallique, le Lithium-ion (Li-ion) ne présente pas de danger si certaines précautions lors de la charge et de la décharge sont prises.


L’immense majorité des véhicules électriques ou hybrides actuels est équipée d’une batterie lithium-ion. Ce type d’accumulateur se trouve également dans les téléphones, ordinateurs portables et de nombreux autres appareils. Ses inventeurs, Stanley Whittingham, John Goodenough et Akira Yoshino, ont été récompensés par le prix Nobel de chimie 2019. Dans les années 1970, en pleine crise pétrolière, Whittingham démontre l’avantage du lithium par rapport aux autres métaux utilisés jusqu’alors (cuivre, platine, nickel…) pour stocker davantage d’énergie avec un encombrement et un poids réduits tout en offrant une durée de vie supérieure. Il crée alors la première batterie au lithium grâce aux financements du pétrolier Exxon. Mais, la crise pétrolière passée, et les coûts de production importants qu’une industrialisation engendreraient conduisent Exxon à arrêter le développement.
En 1980, l’américain Goodenough entreprend de remplacer le sulfure de métal de la cathode par de l’oxyde de cobalt, doublant du même coup les performances de la batterie via une hausse de la tension. Mais la libération rapide des électrons et l’agglomération d’ions de lithium au bout des électrodes, avec un risque de contact et donc de court-circuit, nécessite une stabilisation. Celle-ci arrive en 1985 grâce au japonais Yoshino, qui utilise de la coke de pétrole pour l’anode, qui conduira à la commercialisation de la première batterie li-ion par Sony Corporation en 1991.
Pourquoi les batteries Lithium-ion dominent les usages ?
Aujourd’hui, la technologie des batteries Li-ion permet le plus haut niveau de densité énergétique, jusqu’à 5 fois plus que les batteries Nickel. Les performances telles que la charge rapide jusqu’à 80% de leur capacité, ou la fenêtre de fonctionnement en température (-50°C à 125°C) et leurs usages peuvent être affinés grâce au large choix de conceptions et de chimies des cellules. De plus, les batteries Li-ion présentent des avantages supplémentaires tels qu’une très faible autodécharge, une très longue durée de vie et de grandes performances de cyclage, généralement des milliers de cycles de charge/décharge.
Quelles sont les principales chimies de batteries Lithium-ion ?
Il existe différents types de batteries Lithium, en fonction des chimies utilisées en combinaison. Tout est affaire de compromis. Dans l’automobile, Tesla utilise le NCA et tous les autres le NMC (Nickel Manganèse Cobalt). Dans l’industrie, et notamment pour les bateaux, on retrouve le LTO (Lithium-Titanate, développé par Toshiba) et le LFP (Lithium-Fer-Phosphate) pour leur sécurité, leur durée de vie. Le LFP est plus énergétique que le LTO. Le LTO est fait pour du grand cyclage, de la charge rapide, mais peu d’autonomie — il est cher et lourd. Le LFP sur les bateaux électriques ou hybrides est une bonne solution pour allier sécurité, performance et durée de vie ; moins dense que la technologie Lithium NMC utilisée dans les voitures mais bien plus à l’abri des emballements thermiques menant à des feux de batteries impossibles à éteindre, la technologie LFP se retrouve aussi dans les applications médicales et solaires.
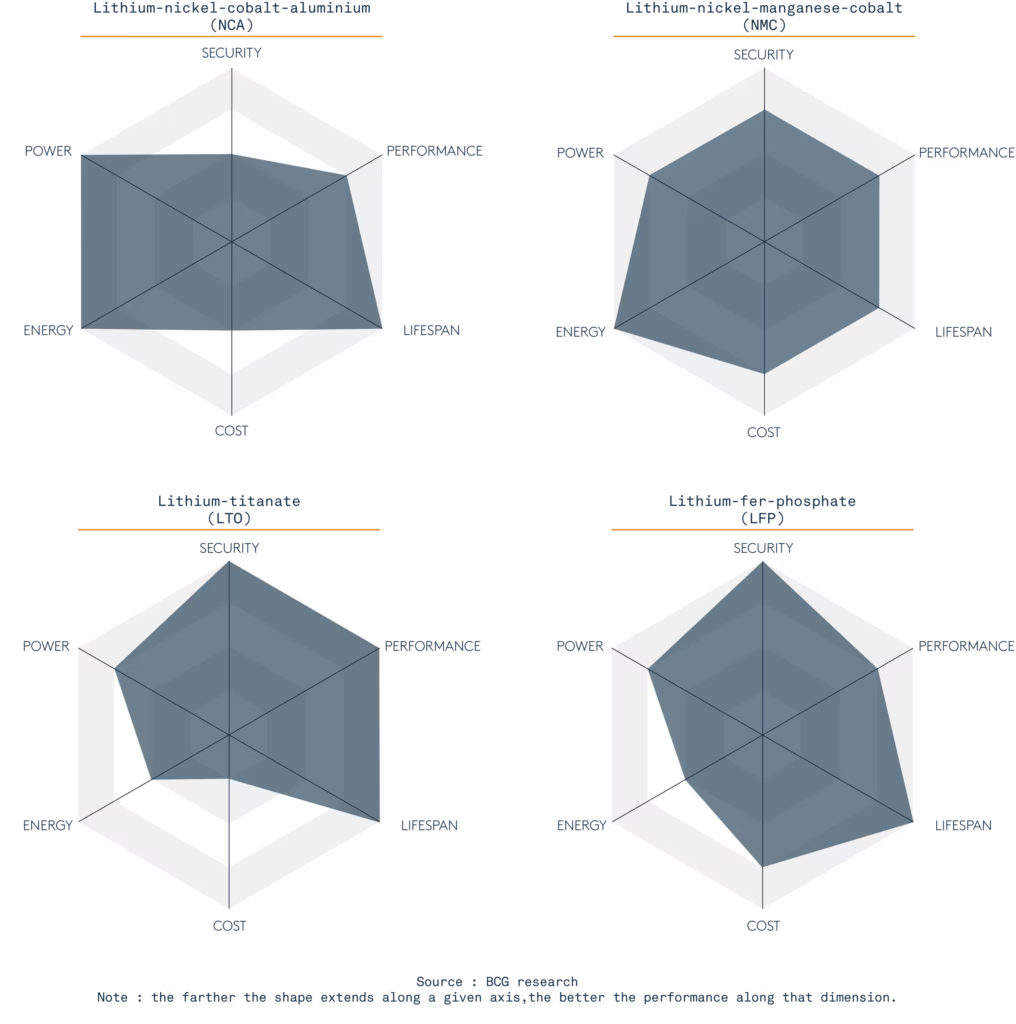
Quelles sont les autres chimies actuelles de batteries Lithium-ion ?
Lithium-polymère (LiPo)
Cette électrochimie est très puissante en décharge et sans effet mémoire mais sa durée de vie demeure courte par rapport à son prix. On retrouve ces batteries dans les applications de l’aéromodélisme et dans les appareils connectés.
Lithium-nickel-cobalt-aluminium (NCA)
Dense en énergie comme en puissance, le NCA est plus économique que le NMC. Néanmoins, il est presque aussi sensible que le NMC ce qui limite son utilisation. Il est très utilisé pour les applications en mobilité électrique ou à grande puissance, comme les véhicules de golf par exemple.
Lithium Manganèse (LMO)
Ici le cobalt est remplacé par le manganèse, rendant ainsi cette technologie plus durable et plus économique. Néanmoins sa durée de vie est faible avec en moyenne entre 300 et 700 cycles.
Quelles sont les nouveautés ?
De nouvelles technologies électrochimiques se développent ces dernières années :
- Lithium-cobalt-oxide (LCO) : Composée de cobalt, cette technologie a un coût très élevé et peut être très dangereuse. Néanmoins elle demeure significativement capacitive.
- Lithium-soufre : Encore en cours de développement, ces batteries sont légères, économiques et très capacitives. Le seul problème : leur faible durée de vie.
- Lithium-métal-polymère (LMP) : Plus facile à recycler, le LMP a néanmoins une durée de vie très faible pour une capacité moyenne.
- Lithium-air : Dense en énergie, cette technologie est encore en développement car elle est très corrosive.
La fabrication des batteries pose souvent question: recyclabilité, rareté des métaux… qu’en est-il vraiment?
La plupart des matériaux ne sont pas si rares que ça, pas si polluants non plus, surtout pour la chimie LFP. Du lithium, du phosphate de Fer, de l’aluminium, du cuivre. Pas de Nickel, pas de Cobalt, pas de Manganèse, pas de Plomb, pas de Cadmium, pas de métaux lourds, pas de colles, pas de fluide de refroidissement, pas de résines…
La filière de recyclage se met en place. Aujourd’hui, elle existe à des niveaux préindustriels, mais le coût du recyclage est déjà très bas (1€/kg) et va continuer de baisser avec l’augmentation des volumes. Le recyclage permet de récupérer les métaux pour les réutiliser.
Certaines batteries sont totalement démontables, et leur recyclage permet de trier en amont les composants pour soit les réutiliser, soit diminuer le coût de traitement. Si l’on prend comme exemple une batterie LFP telle que celles développées par EVE System (partenaire d’EODev), elle a une empreinte carbone de moins de trois tonnes de CO₂ sur la totalité de l’Analyse de son Cycle de Vie. L’équivalent d’environ 10.000 kms parcourus avec une voiture ou d’un camion Diesel qui consommerait 10L au 100 kms. Ce qui, à 100 km/h de moyenne sur autoroute, correspond à une centaine d’heures d’utilisation, contre plusieurs centaines, voire milliers de cycles pour une batterie Li-ion.


Comment sont certifiées les batteries pour les usages maritimes ?
Le secteur maritime impose, en fonction de la taille des bateaux, de se conformer aux règles de certification marine des installations. Cela passe par des « approbations de type » sur les composants, et des revues de conception plus ou moins poussée par les organismes certificateurs. Cette « certification de type » concerne tous les organes de sécurité du navire, comme les moteurs, les arbres, les hélices, les réducteurs, les variateurs, les batteries, les commandes de gaz, les disjoncteurs, les câbles, etc. La certification marine des batteries consiste à répondre à des tests environnementaux particuliers (vibration, chaleur humide, chaleur sèche), des tests fonctionnels, des tests normalisés de performances (IEC 62619 et IEC62620). Par rapport aux normes industrielles classiques, le niveau de tenue aux vibrations est supérieur, et le test de chaleur humide est drastique.
