Did you know?
The EODev industrialization ecosystem relies on a combination of forward thinking yet mature technologies that are turned into new products and solutions as our Research & Development team strives to bring to market the most efficient and reliable energy solutions. Some of these technologies have existed for many years, some others have only recently reached readiness levels that make them relevant for widespread applications. But all of them participate in the energy transition towards a world less impacted by human beings, with the lowest carbon footprint possible.

Hydrogen
Hydrogen is the simplest, lightest atom – since it consists of a nucleus containing a proton and a peripheral electron – and the most abundant in the universe: It represents 75% of the mass of the universe and two thirds of all molecules on our planet. Fuel for the stars and fuel for the “empty spaces” between the stars, hydrogen is not a primary energy but an energy carrier. Unlike other sources, such as fossil or renewable energies, it is a secondary energy without CO₂ emissions.

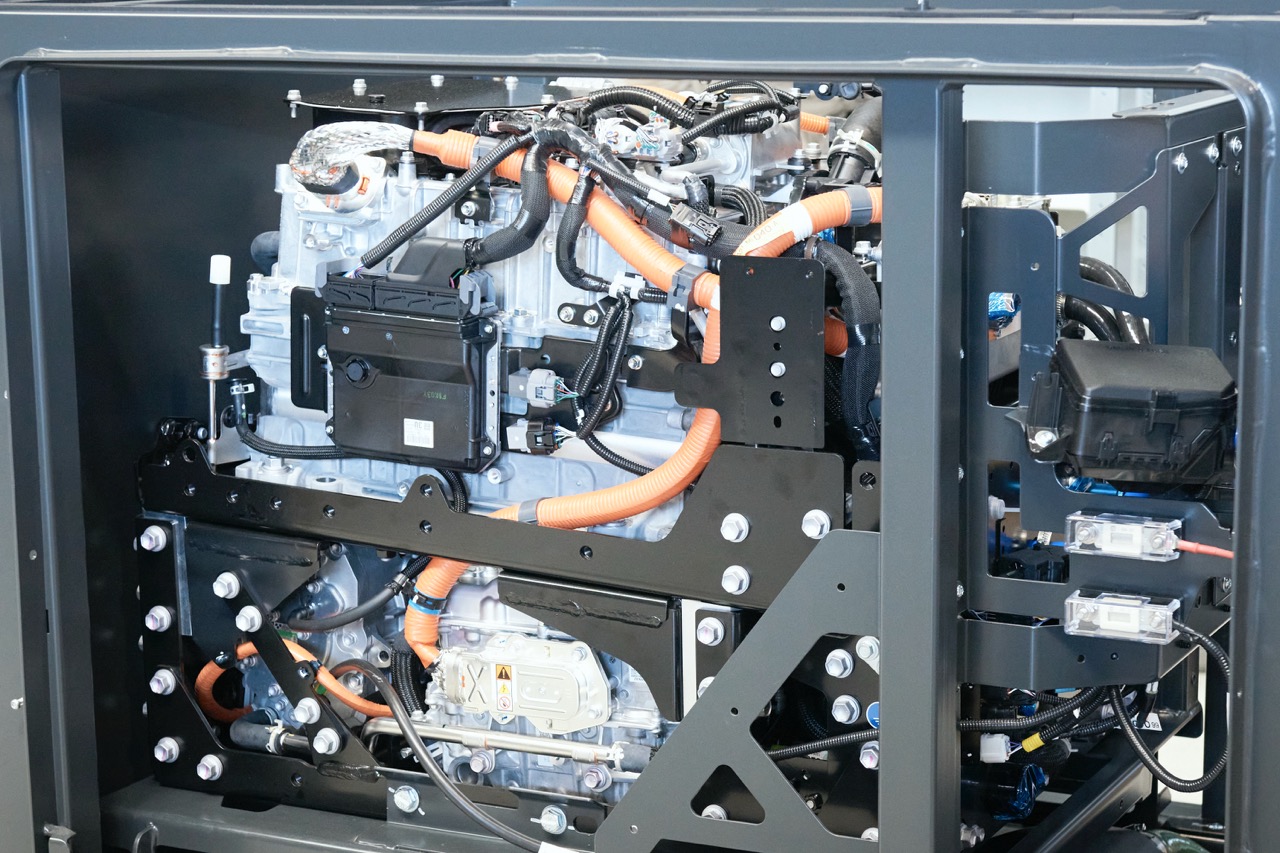
Fuel Cell
A fuel cell is made of metal, graphite, electrodes and its process is chemical. A fuel cell converts chemical energy (energy stored in molecular bonds) into electrical energy. A PEM (Proton Exchange Membrane) cell uses hydrogen gas (H₂) and oxygen gas (O₂) as fuels; the generation of electricity will take place via the oxidation of a reducing fuel, hydrogen, and the reduction of an oxidant such as the oxygen contained in the air. The reaction products in the cell are water, electricity and heat. As oxygen is readily available in the atmosphere, it suffices to supply the fuel cell with hydrogen which can come from an electrolysis process.
Electro-hydrogen hybridization
Electro-hydrogen hybridization is at the heart of the propulsion system of The New Era from Hynova Yachts, the first “dayboat” equipped with a hydrogen-powered REXH₂ . The operation of such a system has paved the way for carbon-free maritime mobility with greater autonomy.
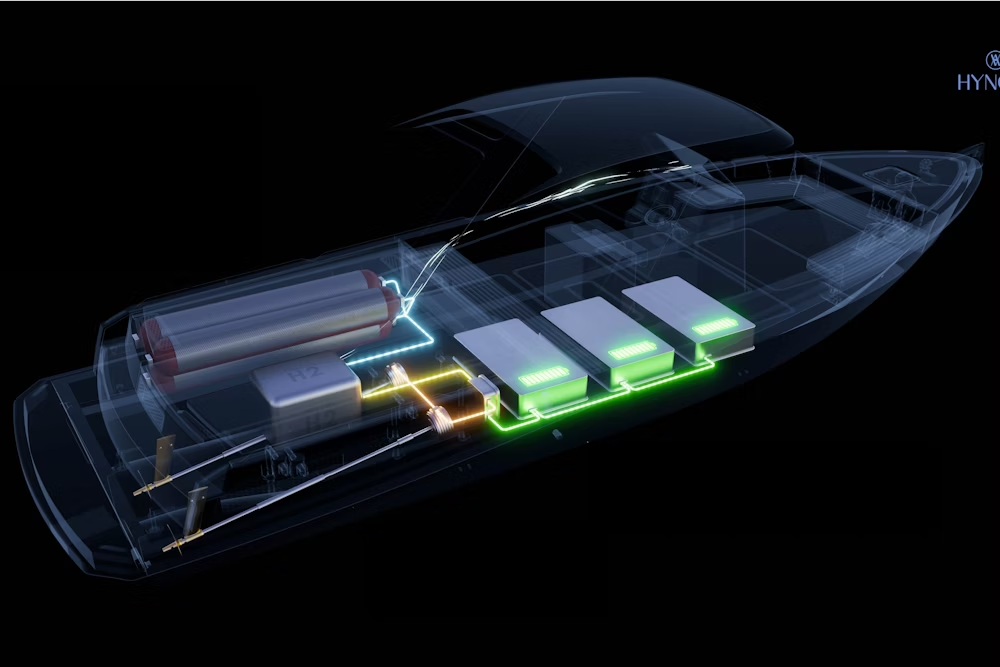

Batteries
Batteries are made up of accumulators. Batteries have the particularity of storing energy and being able to be recharged. They comprise cells, each equipped with a positive electrode (the cathode), a negative electrode (the anode), a separator and an electrolyte which delivers electrical energy by changing state. Depending on the chemical components and the materials used for these elements, the properties of the battery are different and have an impact on the quantity of energy stored and delivered, the power supplied as well as the number of possible charge and discharge cycles. Battery manufacturers are constantly looking for more economical, denser, lighter and more powerful electrochemical systems.
